QT Medical Receives CE Mark for Home-Use 12-lead ECG
2020-06-05QT Medical strives to make hospital grade 12-lead electrocardiograms (ECG) accessible to everyone everywhere.
Los Angeles, CA – June 4, 2020 – QT Medical, a leader in electrocardiogram (ECG) technology, has once again reached another major milestone by receiving it’s CE Mark on its flagship product, PCA 500. In addition, QT Medical has also received ISO 13485:2016 certification as a manufacturer of high-quality medical devices by conforming to strict guidelines and high standard QMS practices.
The resting 12-lead ECG is the most commonly used test for the heart. QT Medical strives to make this useful tool available for patients to use at home. Focusing on innovations that advance telehealth and home care, QT Medical offers a revolutionary solution for managing heart health anytime, anywhere.
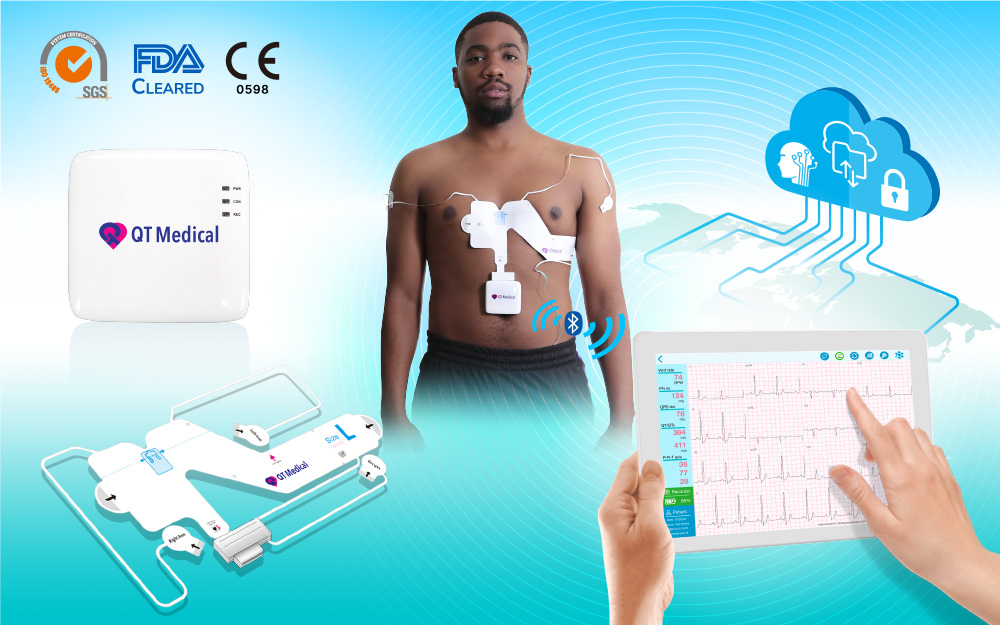
PCA 500 is the only hospital quality, medical standard 12-lead ECG that can be used by patients at home. Cleared by the FDA in 2018, PCA 500 has been used in medical clinics and clinical trials in the U.S., and has been successfully tested in major airlines. With CE Mark, PCA 500 will soon be available in the EU and many nations in the Middle East and Southeast Asia.
“This is definitely a market disruptor,” said Dr. Ruey-Kang Chang, CEO of QT Medical. “There are many single-lead ECGs like the Apple watch and AliveCor, but their clinical use is limited to heart rate and some arrhythmias. PCA 500 is the medical standard 12-lead ECG that can detect a full range of heart diseases, same as the ECG tests done by technicians in hospitals. To really democratize ECG in the midst of the COVID-19 pandemic, we are making PCA 500 accessible and affordable with a new service—Xpress ECG.”
Xpress ECG is an online order mail delivery service, where the physicians place the order, and QT Medical sends the ECG kit which includes everything the patients will need to complete the test in the comfort of their own homes. Xpress ECG service was launched in the first week of June 2020, and many cardiologists, electrophysiologists and primary care physicians have registered to use the service.
In the COVID-19 pandemic, patients with heart disease have a much higher risk of infection and mortality. Using Xpress ECG, we can minimize the potential risk of exposure in these patients. Using PCA 500 in clinics also helps to reduce the risk of disease transmission between patients from contaminated ECG leadwires and electrodes.
With the clearance of FDA and now CE Mark, we are bringing this disruptive innovation to the huge market of cardiovascular diseases care worldwide. Currently, QT Medical is working on obtaining FDA clearance for pediatric use, which is expected later this year. AT QT Medical, our mission is to improve heart health for everyone, everywhere, through superior ECG and AI technology.
ABOUT PCA 500
PCA 500 is hospital-quality, medical standard 12-lead ECG that can be operated by patients at home with no need for training. The PCA 500 platform includes a super-compact ECG recorder, a proprietary pre-positioned electrode strip that comes in 4 sizes for adults, apps for iOS and Android phones and tablets, computer interpretation, and HIPAA-compliant cloud.
For more information:
https://www.qtmedical.com/index.html#QTECG
ABOUT Xpress ECG
Xpress ECG is an online order mail delivery ECG service. You create an online account with no cost and no obligations. When your patients need an ECG test, you can order the test from your online dashboard. We send the ECG kit to the patient’s home by mail. The patient completes the test with an easy to use electrode and a wireless recorder. Upon completion, the ECG is sent to the cloud, and the recorder is mailed back. You can review the results instantly, and send a copy of the final report to the patient right from the online dashboard.